Home
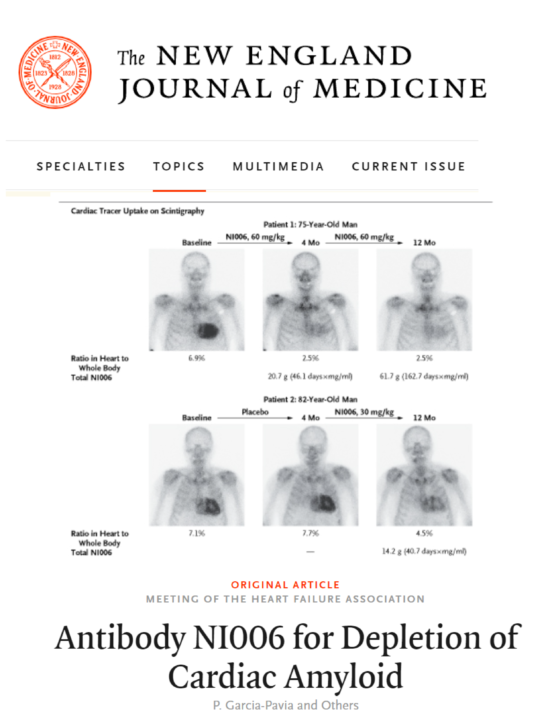
Neurimmune is a biopharmaceutical company translating human immune memory into antibody therapeutics. Neurimmune’s pipeline comprises drug candidates for Alzheimer’s disease, amyotrophic lateral sclerosis, fronto-temporal dementia as well as cardiomyopathy and type 2 diabetes.
About Neurimmune