Discovery of aducanumab
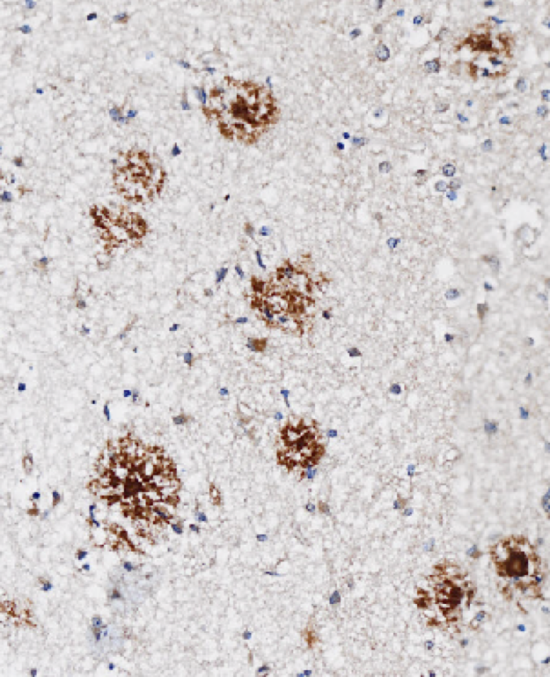
Alzheimer’s disease - the leading cause of dementia in elderly people - is characterized by a decade-long build-up of a protein in brain known as amyloid. It can damage the brain’s nerve cells resulting in progressing deficits in memory, learning, orientation in space and time, language, and thoughtful planning. Amyloid is readily detectable in affected brains and its removal is a therapeutic objective in Alzheimer’s disease.
Aducanumab removes amyloid from brains (Sevigny J. et al., 2016). It is a human monoclonal antibody discovered with Neurimmune’s Reverse Translational Medicine technology. Neurimmune originally licensed aducanumab for the treatment and prevention of Alzheimer’s disease to Biogen in 2007 and regained global rights to the program in 2024. Aducanumab was granted FDA accelerated approval for the treatment of Alzheimer’s disease.
Research at Neurimmune, in collaboration with the University of Zurich, led to the identification of protective anti-amyloid antibodies in healthy elderly people and patients with slowly progressing dementia. These antibodies bound brain amyloid in patient tissues. Study of the antibodies resulted in the discovery of aducanumab. Following intravenous administration, aducanumab crosses the blood-brain-barrier, binds to brain amyloid, and removes it with the help of the immune system.